Table of Contents
Introduction to Antipsychotics
Primer
Antipsychotics (also known as neuroleptics or major tranquilizers) are a class of medications mainly used in the treatment of psychosis in schizophrenia and psychosis/mania in bipolar disorder. Its use has expanded to neurodevelopmental, neurodegenerative, and neuropsychiatric disorders in recent years.
History
- The first antipsychotic chlorpromazine was introduced in 1955 by French psychiatrists Pierre Deniker and Jean Delay. Since then, antipsychotics have been the cornerstone treatment for psychotic disorders, and considered the single greatest advance in the treatment of mental disorders.
- Its role and scope has expanded in the treatment of other medical and psychiatric illnesses since then.
The Dopamine Hypothesis
One theory in the pathophysiology of schizophrenia is that increased dopamine activity causes the positive symptoms of schizophrenia. Similar to how methamphetamine and cocaine also increases dopamine activity, and can cause schizophrenia-like symptoms. Therefore, antipsychotics target the mesolimbic pathway to decrease the incidence of positive symptoms. Antipsychotics work by binding to dopaminergic neuroreceptors. It is important to keep in mind that this is a theoretical model, and that the pathophysiology of schizophrenia remains poorly understood.
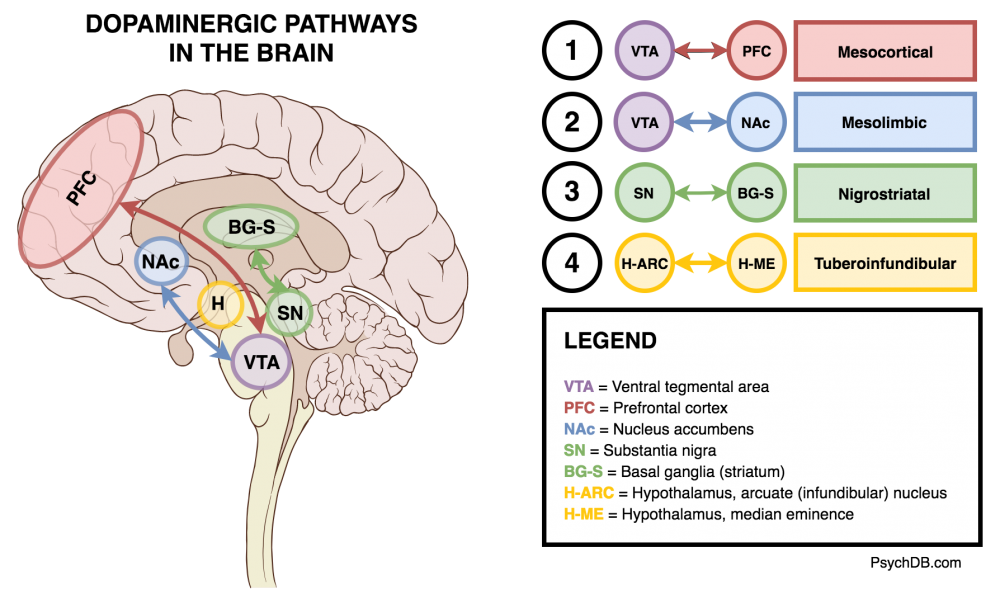
Dopaminergic Pathways
There are 4 key dopaminergic pathways that antipsychotics affect (See also: The Four Dopamine Pathways Relevant to Antipsychotics Pharmacology).
The Four Key Dopamine Pathways
| Pathways | Projection | Relevance |
|---|---|---|
| Mesocortical | Ventral tegmental area → prefrontal cortex | Decreased dopamine leads to negative symptoms (amotivation, flat affect, anhedonia). Cognitive symptoms are also thought to be due to a hypoactive mesocoritcal pathway. |
| Mesolimbic (reward) | Ventral tegmental area → nucleus accumbens and olfactory tubercle. | Increased dopamine leading to positive symptoms (hallucinations, paranoia, delusions). Importantly, the mesolimbic pathway is the same pathway for nicotine-reward pathway. |
| Nigrostriatal | Substantia nigra → striatum (caudate and putamen) | This pathway also gets blocked by antipsychotics, leading to extrapyramidal symptoms (EPS), such as tremors, slurred speech, dystonia, and other abnormal movements. Akathisia is another side effect. |
| Tuberoinfundibular | Entirely in hypothalamus, from arcuate (infundibular) and periventricular nuclei to the median eminence. | The decrease of dopamine in this pathway, through the use of antipsychotics, can cause an increase prolactin (hyperprolactinemia), leading to side effects like gynecomastia, galactorrhea, and menstrual irregularities. The regular role of dopamine release in the tuberoinfundibular pathway is to tonically inhibit prolactin release. |
Mechanism of Action
Receptor Occupancy
The therapeutic action of an antipsychotic occurs when 65% to 85% of brain dopamine (D2) receptors are occupied.[1] When more than 80% of the dopamine (D2) receptors are occupied, hyperprolactinemia and parkinsonism can result. In addition to percentage occupancy, the duration of time that the antipsychotic drug stays attached to the D2 receptor impacts the degree of extrapyramidal symptoms (EPS). For example, haloperidol remains attached to the D2 receptor in vitro for over 38 minutes. Atypicals like clozapine and quetiapine dissociate from the D2 receptor quickly, in about 15 seconds (this has been referred to the “kiss and run” hypothesis).[2][3] As a result, these rapidly dissociating atypical antipsychotics have a lower incidence of EPS. Of note, the serum level of antipsychotics do not correlate with brain occupancy due to the blood brain barrier.
Potency
Antipsychotic function can best be understood by their degree of affinity for the dopamine receptor, or potency (i.e. - “how much of the drug you need to produce a clinical effect?”).[4][5] This can be seen in the actual dose of the antipsychotic itself (e.g. - the therapeutic dose of risperidone (high potency) is 2-3 mg, whereas quetiapine (low potency) can be up to 800 mg). The potency of an antipsychotic in particular can tell you its particular effects and side effects. For example, all low potency antipsychotics (regardless if it is an FGA or SGA) have a lower incidence of EPS. This is because all low potency antipsychotics are also more anticholinergic (i.e. - in addition to targeting the D2 receptor, they also have greater anti-muscarinic activity). This means low potency antipsychotics have “built-in” benztropine (an anticholinergic medication used in the treatment of EPS). Understanding the pathophysiology of EPS will also help one understand why benztropine is given, and why EPS occurs with high potency antipsychotics.
- High potency (i.e. - you only need to prescribe a “low dose” to get a clinical effect)
- These antipsychotics are much more prone to producing extrapyramidal symptoms due to their higher affinity for the dopamine (D2) receptor. Hyperprolactinemia is also a side effect of potent dopamine blockade. These side effects are more common in first generation antipsychotics, but also applies to second generation antipsychotics. High potency antipsychotics are also relatively less sedating (compared to low potency), because there is a lower affinity for the histamine (H1) receptor.[6]
- Medium potency (i.e. - intermediate effects in terms of sedation, anticholingeric side effects, and EPS)
- Low potency (i.e. - you need to prescribe a “high dose” to get a clinical effect)
- These antipsychotics are much more anticholinergic and thus more sedating and causes constipation. Cardiovascular side effects also arise due to adrenergic receptor blockade. However, this equates to fewer extrapyramidal symptoms.
Antipsychotic Potency Table
| Potency | First Generation (Typical) | Second Generation (Atypical) |
|---|---|---|
| HIGH | Haloperidol | Risperidone |
| Flupenthixol | Paliperidone | |
| Fluphenazine | ||
| Pimozide | ||
| MEDIUM | Loxapine | Olanzapine |
| Zuclopenthixol | Ziprasidone | |
| Perphenazine | Aripiprazole | |
| LOW | Chlorpromazine | Quetiapine |
| Methotrimeprazine | Clozapine | |
| Amisulpride |
Metabolism
- Most antipsychotics are metabolized by CYP 2D6 and 3A4.
- Clozapine and olanzapine (second generation antipsychotics) are also metabolized by CYP1A2.
- There is increased metabolism in Mediterranean populations.
Antipsychotic Classes
First (Typical) vs. Second (Atypical) Generation
The first antipsychotics in the 1950s included chlorpromazine and haloperidol, and were called first-generation antipsychotics (FGAs), or typical antipsychotics. Starting in the 1990s, second-generation antipsychotics (SGAs) were released and marketed. SGAs, also called atypical antipsychotics, were initially thought to be more effective and have a better safety profile. However, the landmark CATIE trial later showed that SGAs were no more effective than FGAs, and that the benefits were mainly promoted by pharmaceutical companies.[8] At the time of their release, it was also thought that SGAs would cause less extrapyramidal symptoms (particularly tardive dyskinesia). This was also another initial reason behind categorizing antipsychotics as either typical (first generation) or atypical (second generation).
Thus, generally speaking:
- FGAs have higher dopamine (D2) receptor occupancy and therefore have a higher risk of causing EPS such as tardive dyskinesia
- SGAs usually have lower dopamine (D2) receptor occupancy, which results in lower incidence of EPS. These would include antipsychotics such as clozapine and quetiapine
- However, distinction becomes blurred when considering higher potency SGAs which can also have moderate to high EPS effects. For example, risperidone at low doses (up to 3mg) does not cause EPS, and behaves like an SGA. But at higher doses (up to 16mg), it acts like an FGA, much like haloperidol, and the risk of EPS becomes equal
- SGAs also have greater antagonism for the serotonin receptor (5-HT2A) than the dopamine receptor (D2), and is why some SGAs, such as aripiprazole and quetiapine, are used in the treatment of mood disorders such as major depressive disorder and bipolar depression
At the present time, the difference between first and second generation antipsychotics is based more on historical and pharmaceutical influence, rather than a black and white distinction. It is more important to look at an individual antipsychotic's potency, receptor profile, and pharmacokinetics, rather than if it is an FGA or SGA.
Treatment
Efficacy
Antipsychotics have a relatively quick onset of action and patients begin responding within 1 week.[9] Patients usually first have improved sleep due to the sedating effects of antipsychotics, followed by decreased anxiety occurs from resolution of the psychotic symptoms. Despite pharmaceutical company marketing, there is no evidence that first and second generation antipsychotics differ in efficacy. The only exception to this is clozapine, which is the gold-standard medication for treatment-resistant schizophrenia, and has the lowest re-hospitalization rate compared to all other antipsychotics.
w
Length of Treatment
- A recent study observed that compared with a standard maintenance treatment regimen, dose reduction or supervised discontinuation of antipsychotic medication during the early phases of FEP led to a higher relapse rate initially, but improved long-term outcomes. This study has been criticized for its unequal distribution across diagnostic groups, high attrition rate, failure to separate the dose reduction and discontinuation groups, and the fact that most patients in each arm of the study still did receive medication.[13]
- Other studies have suggested that up to 40% of first episode psychosis patients are able to achieve good outcomes with either low or no doses of antipsychotics.[14]
- Yet other retrospective studies have shown that more breaks in antipsychotic treatments may result in greater risk of relapse and longer time to remission.[15]
- Practically speaking, however, clinicians and researchers are still unable to discern which populations will do well with an antipsychotic taper, and those who would worsen symptomatically.
- The 2023 RADAR Trial found that slow, supported dose reduction of antipsychotics was associated with a higher risk for relapse and did not result in improved social outcomes.[16]
- Relapse involves hospitalization, recurrent psychosis, and significant psychosocial impact, and this needs to be weighed carefully against discontinuation of medication.
- Thus, long-term antipsychotic use for treating first-episode schizophrenia for the majority of patients remains the most evidence-based practice, as the relapse rates are greater than 80% with medication-discontinuation after several years.
Long-Acting Injectables
The literature shows that clozapine and long-acting injectable antipsychotic medications have the highest rates of prevention of relapse in schizophrenia.[20] The risk of rehospitalization is about 20% to 30% for patients treated with long-acting injectable treatments compared with the oral equivalents.[21] If a patient is doing well on an existing first-generation long-acting injectable, switching them to a second-generation LAI puts them at a higher risk of treatment discontinuation and weight gain.[22]
Polypharmacy
Antipsychotic polypharmacy (i.e. - taking more than 1 antipsychotic) is commonly seen, but really should not occur! Generally speaking, patients with antipsychotic polypharmacy should be transitioned to monotherapy treatment. Furthermore, changing to monotherapy does not compromise the clinical response.[23] One recent cohort studies did show superiority of clozapine plus aripiprazole over clozapine alone.[24] However, antipsychotic polypharmacy should always be a treatment of last resort.
Switching/Tapering
When switching from one antipsychotic to another, there is no difference in clinical outcomes (psychopathology, extrapyramidal symptoms, and adverse effects) between maintaining the patient on the old antipsychotic for a period of time and starting the new antipsychotic, versus stopping the old antipsychotic immediately and introducing the new antipsychotic.[25]
Discontinuation
Discontinuation of antipsychotics is often a patient request due to intolerable side effects from the medication. This can present as a challenging clinical decision.[26] Rapid or abrupt antipsychotic discontinuation is one of the major reasons for symptom recurrence and readmission to hospital. Therefore, any discontinuation should be carefully monitored and tapered slowly. The abrupt withdrawal of clozapine has been associated with cholinergic rebound and rapid onset of psychosis. Discontinuation-induced supersensitivity of dopamine receptors can lead to worsening and the appearance of new and more complex symptoms.[27] Therefore, clozapine should always be gradually and slowly tapered over time.
Monitoring
All patients who are on long-term antipsychotics must be medically monitored routinely.
Antipsychotic Monitoring
Adapted from: Pringsheim, T. et al. (2017) Physical health and drug safety in individuals with schizophrenia. The Canadian Journal of Psychiatry, 62(9), 673-683.| Initiation | 1 month after initiation | 3 months after initiation | Then annually | |
|---|---|---|---|---|
| Electrolytes, Cr, LFTs, TSH | ✓ | As clinically indicated | As clinically indicated | As clinically indicated |
| Fasting plasma glucose | ✓ | As clinically indicated | ✓ | ✓ |
| HbA1c | ✓ | - | ✓ | ✓ |
| Lipid panel (total cholesterol, LDL, HDL, triglycerides) | ✓ | As clinically indicated | ✓ | ✓ |
| Body mass (BMI) | ✓ | ✓ | ✓ | ✓ |
| Blood pressure (BP) | ✓ | As clinically indicated | ✓ | ✓ |
| Extrapyramidal symptom (EPS) exam | ✓ | ✓ | ✓ | ✓ |
| Endocrine function history (gynecomastia, galactorrhea, libido) | ✓ | - | ✓ | ✓ |
| Prolactin | If clinically indicated | If clinically indicated | If clinically indicated | If clinically indicated |
| ECG (QT monitoring) | If clinically indicated (some clinicians will order this routinely as a baseline) | - | If on multiple QTc-prolonging medications (or if clinically indicated) | As clinically indicated, or yearly |
| Smoking history | ✓ | - | ✓ | ✓ |
Side Effects and Adverse Events
Antipsychotics are not benign, and must be used judiciously. The main side effects include extrapyramidal symptoms and sexual side effects,[28] (due to potent dopamine receptor blockade), anticholingeric side effects like sedation (from blockade of histaminergic and muscarinic receptors), and cardiovascular (from anticholinergic effects and adrenergic blockade).[29] Adverse reactions include neuroleptic malignant syndrome, seizures, and cardiovascular problems. The reason for many of the side effects associated with antipsychotics is because antipsychotics do not exclusively target the dopamine receptors, and other receptors are also targeted.
Side Effects
Side Effects Associated with Antipsychotics
| Side Effects | Description | Management |
|---|---|---|
| Metabolic Syndrome | Atypical antipsychotics can cause hypertension, high blood sugar, obesity, and abnormal cholesterol or triglyceride levels, resulting in metabolic syndrome. Patients on atypical antipsychotics should be routinely monitored with metabolic blood work. | See main article |
| Constipation | Chronic antipsychotic use can lead to constipation, which can be severe. Constipation can be a serious and life-threatening issue for patients on clozapine. | First-line management and treatment includes the use of an osmotic agent, such as polyethylene glycol (PEG), and/or a stimulant laxative such as senna. There is limited evidence for the use of surfactant agents (“stool softeners”) like docusate sodium for chronic constipation. These agents only lower the surface tension of stool, but are not effective at preventing and treating medication-induced constipation. Patients on clozapine need to have regular bowel monitoring as the risks of complications are higher! |
| Sedation | Certain antipsychotics are more sedating than others. The degree of sedation depends on the affinity to the histamine (H1) receptor. Generally, low potency antipsychotics are more sedating than high potency ones, and atypicals are more sedating than typicals. The most → least sedating antipsychotics (by H1 receptor affinity) are: clozapine > olanzapine > quetiapine > risperidone.[30] | Reduce dose or consider antipsychotic switch to a higher potency antipsychotic. |
| Hyperprolactinemia | Hyperprolactinemia can be completely asymptomatic or be very distressing to patients when they experience associated symptoms including: menstrual disturbances, anovulatory cycles, galactorrhea, impaired fertility, or sexual dysfunction. It is due to the blockade of the tuberoinfundibular pathway. | See main article |
| Amenorrhea | In females, hyperprolactinemia can result in amenorrhea, potentially causing ovarian dysfunction or infertility. | Metformin can restore menstruation in obese women with amenorrhea by restoring sex hormone levels and decreasing insulin resistance.[31] Asking for a menstrual history is thus important for females on antipsychotics. |
| Sexual Dysfunction | Sexual dysfunction is common condition in patients taking antipsychotics, and caused by hyperprolactinemia and dopamine blockade.[32] It is also the most bothersome symptom for patients. Sildenafil can be prescribed as a treatment for sexual dysfunction. | See main article |
| Hypotension | Orthostatic hypotension can be common in atypical antipsychotics (ranging from paliperidone to clozapine). It is thought to be caused by anticholinergic effects and/or alpha-1 adrenoceptor blockade.[33] | Consider adjusting the dose, increasing hydration, or change to a less anticholinergic antipsychotic (i.e. switching from low potency to high potency) |
| Tachycardia | Tachycardia can result from low potency antipsychotics that have a higher anticholinergic burden. | Consider adjusting the dose, or changing to a less anticholinergic antipsychotic. In severe cases, tachycardia can be treated with beta‐blocker |
| QTc Prolongation | Antipsychotic increase the risk of ventricular arrhythmias, through the blockade of potassium channels and prolongation of the QT interval (i.e. - cardiac re-polarization). Other mechanisms may be involved, including autonomic effects and inhibition of other ion channels. Clozapine is also associated with myocarditis, which is not seen with other antipsychotics.[34] | See main article |
| SIADH and Hyponatremia | In rare cases, antipsychotics can cause hyponatremia.[35] Antipsychotics are thought to increase AVP release is increased despite normal plasma osmolality, resulting in syndrome of inappropriate antidiuretic hormone secretion (SIADH), which can result in hyponatremia. | See main article |
| Sialorrhea (Hypersalivation) | Hypersalivation/sialorrhea is a common side effect related to clozapine use (but can also occur in other antipsychotics). Sialorrhea can range from being mildly uncomfortable drooling to potentially life-threatening conditions, such as parotitis, choking, and aspiration. | See main article |
Adverse Events
Adverse Events Associated with Antipsychotics
| Side Effects | Description | Management |
|---|---|---|
| Extrapyramidal Symptoms (EPS) | Extrapyramidal side effects include acute dystonia, akathisia, neuroleptic-induced parkinsonism, tardive dyskinesia, and tardive dystonia. | See main article |
| Neuroleptic Malignant Syndrome (NMS) | NMS is a life-threatening idiosyncratic reaction to antipsychotics characterized by fever, altered mental status, muscle rigidity, and autonomic dysfunction. It is hypothesized to be due to excessive dopamine receptor blockade. Certain individuals may be at a higher risk for NMS. | See main article |
| Seizures | Both typicals and atypicals can lower the seizure threshold. With typicals, chlorpromazine has the highest risk of inducing seizures, while haloperidol, fluphenazine, pimozide and trifluoperazine are associated with a lower risk of seizure. With atypicals, clozapine is commonly associated with seizures, while risperidone has the lowest risk.[36] | - |
Special Populations
Special Populations
| Population | Considerations |
|---|---|
| Elderly | All antipsychotic agents are associated with increased risk of mortality in elderly patients with dementia.[37] There is also an increased incidence of cerebrovascular events (e.g. TIAs, stroke) has been seen among elderly patients on antipsychotics. When benefits outweigh risks, use lower doses, slower titration schedules, and periodic reassessment for the necessity of the medication |
| Pediatric and Youth | Children and youth who are on second generation antipsychotics must have close metabolic monitoring. |
| Renal Impairment | All first generation antipsychotics can be used with caution in those with renal impairment without dosage adjustment. However, recommend initiation at lowest dose (especially in patients with concurrent hepatic impairment). Second generation antipsychotics must be carefully titrated.[38] |
| Hepatic Impairment | No dosage adjustment necessary for use of the first generation antipsychotics. However, recommend initiation at lowest dose (especially in patients with concurrent renal impairment). Asenapine, lurasidone, paliperidone, and risperidone should be avoided in those with severe hepatic impairment.[39] |
| Pregnancy | Only clozapine and lurasidone are FDA Risk Category B (safe for use in pregnancy, but limited human data available; use with caution). All other antipsychotics are FDA Risk Category C (not safe in pregnancy, use only if benefit outweighs risk). All antipsychotics are also proven, or presumed, to be excreted into breast milk. Breastfeeding while on antipsychotics is not recommended.[40] |
Controversy
Brain Volume
An initially controversial paper came out in 2011 that suggested antipsychotics were linked to smaller gray matter volumes.[41] Since then, there has been conflicting research on whether this is due to exposure to antipsychotic drugs versus the degenerative process behind psychosis.[42][43] In 2020, the first randomized control trial showed that antipsychotics (olanzapine specifically) changes brain structure. After 36 weeks of olanzapine exposure, individuals experienced 1.2% loss of cortical volume, which is equal to twice the yearly loss of cortical thickness in older adults.[44] However, structural MRI techniques do have inherent limitations, and they may not actually indicate unqualified tissue loss, and the findings from this RCT has also been challenged.[45] Other neuroimaging studies have also shown reductions in brain volume associated with antipsychotic uses over time.[46] Regardless, the results from this study should have clinicians consider the risks and benefits of antipsychotics, especially in patients without psychosis. Based on the research evidence of the last decade, there needs to be more urgent research on the long-term effects of antipsychotics on the brain. Interestingly, other studies have shown that even unaffected first-degree relatives of individuals with schizophrenia also have thinner cortical volume.[47]
Mortality
- There is no significant difference in the incidence of all-cause death and death due to suicide between oral psychotics, injection antipsychotics, and placebo in individuals with schizophrenia.[48] Nation-wide cohort studies have found that antipsychotic use, in particular with clozapine, has led to lower mortality.[49]
- Several population-based studies have shown a risk for increased all-cause mortality in non-elderly adults with depression who are treated with second generation antipsychotic augmentation.[50]